
New: ComASP® Benzylpenicillin Microdilution Panel
Latest Eucast guidelines for meningitis
System for benzylpenicillin susceptibility testing
with broth microdilution method according to ISO 20776-1
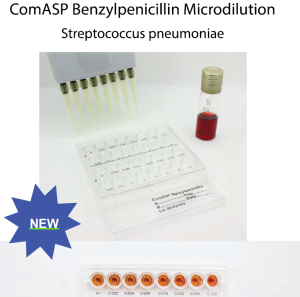
ComASP® Benzylpenicillin 0.002-32 is an in vitro diagnostic product for antimicrobial susceptibility testing (AST)
of clinical isolates based on growth of the test organism in the presence of various concentrations of an
antimicrobial agent.
This ComASP® system is used for quantitative determination of the minimum inhibitory concentration (MIC) of
benzylpenicillin against Streptococcus pneumoniae.
